Relevant functional interfaces included electrical power interfaces, mechanical integration points, thermal coupling, monitoring and control interfaces, as well as safety-related interlocks.
Particular attention was given to interface stability under varying operational and environmental conditions.
System-level constraints such as redundancy requirements, fault tolerance, certification-related considerations and maintainability influenced the overall design space.
These constraints necessitated a holistic approach beyond component-level optimisation.
From the outset, the project was governed by strict constraints related to scalability, manufacturability and operational safety.
Material concepts and synthesis routes were required to be compatible with robust, high-throughput production processes using readily available, non-hazardous and cost-effective chemicals.
Technical development explicitly addressed limitations and trade-offs related to scalability, integration complexity and operational robustness.
These constraints informed design decisions and boundary conditions throughout the project rather than being treated as secondary considerations.
These constraints explicitly excluded synthesis approaches that depend on complex multi-step syntheses and purification, even if such routes are viable at laboratory scale.
Executive management was jointly exercised by the designated Principal Investigators. Adherence to the defined Key Performance Indicators (KPIs) and the quality of the work performed were reviewed in the context of regular project reviews by a major, state-mandated consulting firm with extensive experience in regulated and security-relevant application environments. The reviews were conducted against defined, application-driven technical and operational criteria, ensuring controlled implementation and target-compliant project delivery.
The technical evaluations were performed within clearly defined boundary conditions and assumptions regarding operating profiles, environmental exposure and system integration levels.
This ensured consistency across different development and evaluation activities.
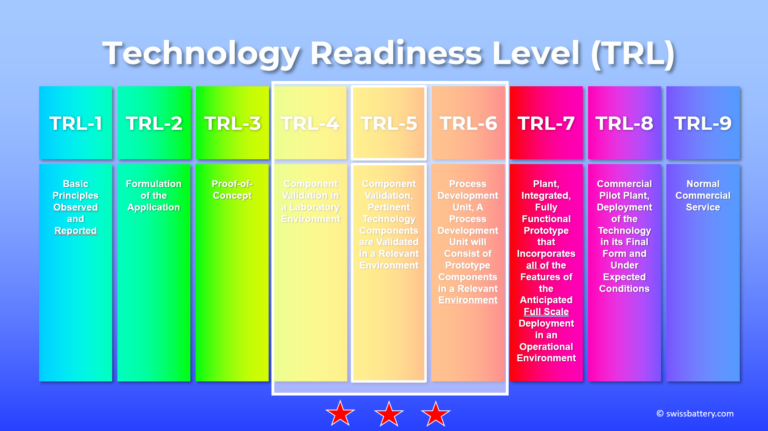
The work was conducted primarily at TRL 3–4 with laboratory-based component validation.
In related programmes, selected elements progressed to TRL 5 and in limited cases towards TRL 6, depending on subsystem and integration level.
In contrast to pharmaceutical drugs, where impurity levels in the range of 1% may often be acceptable, electrochemical energy storage systems exhibit a fundamentally different sensitivity to trace contaminants.
Even minor impurities can lead to severe performance degradation, accelerated ageing or, in extreme cases, complete functional failure of electrochemical devices.
As a consequence, cycle stability and long-term reliability are directly linked to molecular purity, imposing significantly stricter requirements on material quality than in many other application domains such as biotech, pharma and chemistry.
To meet these requirements, the project focused on tailored, highly selective synthesis strategies designed to yield the required molecular quality directly, rather than relying on extensive downstream purification.
This approach is essential for large-scale energy storage applications, where conventional purification methods often become technically impractical or economically prohibitive at the required production volumes.
To assess industrial feasibility, up to twelve CDMOs in Europe and the United States were evaluated and selectively engaged to benchmark scale-up, process robustness and manufacturability constraints.
Experimental and evaluation activities were conducted within a distributed laboratory environment, combining organic synthesis laboratories and battery testing facilities in both academic and industrial settings.
The project was conducted through close interdisciplinary collaboration between organic, analytical and inorganic chemists, electrochemists, physicists and electrical engineers from multiple departments and organizations.
Organic synthesis was performed in two independent preparative-scale organic chemistry laboratories, enabling reproducible synthesis under controlled conditions.
Reaction progress, purity and material consistency were routinely monitored using chromatographic methods complemented by spectroscopic methods.
Electrochemical evaluation was carried out in two battery laboratories, including one industrial partner laboratory and one university-based facility, enabling cross-validation across different testing environments.
Testing infrastructure comprised approximately 30 cycling channels supporting low- to medium-current testing of coin cells and small-format pouch cells for cycle stability and rate capability assessment.
In addition to galvanostatic cycling, approximately 10 impedance measurement channels were available for in-situ electrochemical impedance spectroscopy (EIS), supporting spectroscopic analysis of material behaviour and interface evolution during operation.
Material characterisation and post-test analysis were supported by access to electron microscopy, infrared and UV–Vis spectroscopy, solution-state and solid-state NMR, microbalance-based mass analysis, and chromatographic methods including HPLC, GC and TLC.
These methods enabled correlation between molecular structure, material purity and electrochemical performance.
Evaluation activities included laboratory testing, functional prototyping and selected system-level demonstrations.
Quantitative data were generated internally where required, while public documentation remains deliberately qualitative.
The project generated sustained interest from application-oriented stakeholders and technology evaluation bodies.
Engagements were conducted in a non-public, non-operational context.
*For the purposes of this document, specific elements are addressed in general terms.