
Meanwhile, it is more than 200 years ago that Alessandro Volta demonstrably built the first functioning battery with galvanic cells using several zinc and copper plates. Even though Volta’s column, as it was called at the time, was still large and unwieldy, it paved the way to electrical engineering and many other interesting discoveries. And up to the present day, not much has changed in the functional principle of a battery.
The figure on the right shows a lithium-metal button-cell with an operating potential of around 3 Volts. An operating potential of 3 Volts allows storing much larger amounts of energy (higher energy density) than in older types of batteries.
A battery is an energy storage device in which the stored chemical energy is converted into electrical energy by reduction-oxidation. In principle, these are two electrochemical substances (anode and cathode) with different electrochemical voltage potentials, which are housed in a casing with electrolyte (see the figure on the right side).
A cell is the smallest basic functional unit of a battery, which consists of a compilation of electrodes with active materials, electrolyte, solvent, containers, connections and usually separators.
The capacity of a cell results from the size or weight, the internal structure and the material combination of the electrodes.
A battery management system BMS is a small computer that enables the battery to function properly and monitors its features.
Such are characteristics as the voltages of the individual cells of the batteries, the voltage of the cells under electric load and the internal resistance.
Safety-relevant events such as overcharging, deep discharge, critical high temperatures, short circuits, etc. are also monitored during operation of the batteries.


The term “battery” is used both often as a generic term for i) “energy storage” and ii) as a term for a non-rechargeable energy storage (primary battery).
Whether a non-rechargeable primary battery (e.g., long-term use in watches) or an accumulator (e.g., in electric cars, EV) is used in a device depends on multiple factors.
The question often arises with battery-powered devices, whether the use of rechargeable batteries would not be more sensible. Unfortunately, this question cannot be answered across the board.
Since rechargeable batteries are subject to a certain self-discharge (up to 60% per month). However, it can be said that batteries make sense for consumers with a short duty cycle but a long standby time.
Classic examples of these consumers would be emergency flashlights, clocks, TV remote controls, or wireless sensors for weather stations or smart home systems.
But often every user must decide for himself whether he prefers to use batteries or rechargeable batteries. Batteries make sense for a model of controller that is only used occasionally.
However, if the model is used regularly,(like an electric car or a mobile phone) it is more economical and also more sustainable to use rechargeable batteries in the remote control.
An older example is the lead-acid accumulator, short “Akku” (see picture) and the lithium-ion battery, which is the newest type of rechargeable battery on the market.
Li-Ion cells typically lose about only 0.1 to 1% of their charge per month.
This is one of many reasons why the lithium-Ion battery is used today in electric cars, UAVs, and consumer electronics such as mobile phones, tablets, iPads, iPhones, and notebooks.

The figure on the right side shows the schematic structure of an alkaline manganese battery.
Hint:
In zinc-carbon batteries, the outer cup was made of zinc. Since the zinc electrode decomposes and becomes holey during discharge, these batteries often leaked when they were used up.
In an alkaline manganese batteries, the outer cup is made of metal and the zinc electrode is housed in the core of the battery. This reliably prevents the battery from leaking.


The Swiss Battery Chemistry has a potential greater than 2.5 Volts.
In the case of a 4.5 V flat battery or 9 V block battery, several individual cells are connected in series within the battery in order to obtain the higher voltage level.
In the case of a 4.5 V flat battery, this means four cells (3 x 1.5 V = 4.5 V) and in the case of a 9 V block battery, this means six cells (6 x 1.5 V = 9 V).
Hint:
In a zinc-carbon battery, the outer cup was made of zinc. Since the zinc electrode decomposes and becomes holey during discharge, these batteries often leaked when they were used up.
In an alkaline manganese battery, the outer cup is made of metal and the zinc electrode is housed in the core of the battery. This reliably prevents the battery from leaking.
Since the different substances from which electrodes in batteries can be made have different chemical voltage potentials, the respective battery types also have different nominal voltages (potentials).
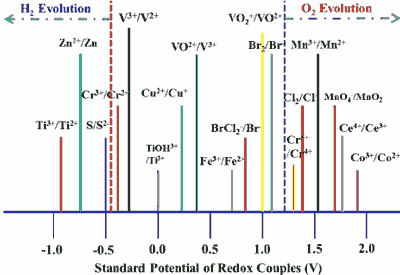

Put simply, in alkaline batteries, the electrical energy is provided by the oxidation of the zinc or the reduction of the copper (or Manganese oxide in alkaline batteries). Since oxidation and reduction take place simultaneously, this is known as a redox reaction. The electrons released in this process are available at the negative terminal of the batteries.
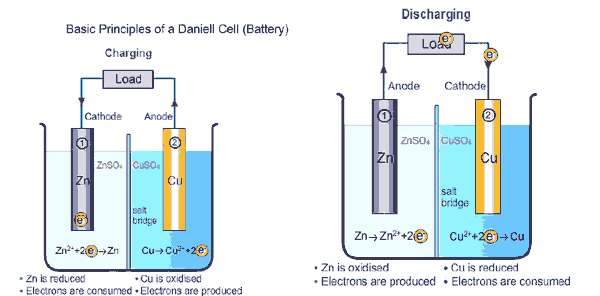
If a consumer device like an electric motor is operated with the battery, the electrons migrate from the negative pole (anode) via the consumer, e.g. the electric motor, to the positive pole (cathode). To balance the charge, ions migrate from the cathode to the anode within the batteries. The chemical processes that take place in the battery during this process are explained obviously in the picture above and in the video attached below.
p.S.: An electric motor in an electric vehicle needs a large assembly of lithium-accumulators (lithium-ion batteries), as they hold much more energy (higher energy density) than all other types of batteries.
Read about the industry lithium-ion battery prospective >>
Want To Learn More
About Our Technology?
Let’s talk
