However, the separator must be permeable to the ions that cause the conversion of the stored chemical energy into electrical energy. The materials used are mainly microporous plastics and nonwovens made of glass fiber polyethylene or polyethylene, which are resistant toward the battery solvents.
Also watch the Battery-Separator video on YouTube
The separator has the function of a barrier that electrically isolates the two electrodes from each other to prevent internal short circuits. At the same time, however, the separator must be permeable to ions so that the electrochemical reactions can take place in the cell.

A separator must be thin so that the internal resistance is as low as possible and a high packing density can be achieved. This is the only way to achieve good performance data and high capacities.
Other important functions of the separator are to absorb the electrolyte and to ensure gas exchange in closed cells. While fabrics and paper were used in the past, very fine-pored materials such as nonwovens and membranes are predominantly used today.
A simple construction of injection-molded plastic webs can also serve as a separator if the only aim is to keep the electrodes at a certain distance apart.
A special form of separator is the tube pocket. This is made from two layers of fabric or non-woven material, which are first impregnated with a resin, then sewn together and formed into a specific tube shape.
These tubes are filled with active mass and are then used as electrodes in lead-acid batteries.
Different separators must also be used for different chemical systems. Their composition depends on the electrolyte to which they are exposed during their service life.
Another criterion for separator selection is price. For example, Utracapacitor-Separators have to be very inexpensive as the stored energy/m2 is very low compared to the energy stored in Lithium-Ion batteries.
Separators that must be stable over many charge/discharge cycles or several years are made of higher-quality materials than those used in short-lived primary cells.
Microporous membranes are used in Lithium-Ion Batteries to enable the passage of ions.
These are usually polymer films, which can also consist of several layers. Since these films have excellent electrochemical properties but only low-temperature resistance (about 115 °C), heat-resistant microporous ceramic separators are also used.
However, their mechanical properties (fracture sensitivity) severely limit their use to exclusively stationary energy storage applications.
Since about 2010, work has been underway on materials based on a very fine nonwoven fabric that has been coated with ceramics.
This achieves a high level of safety through flexibility and temperature resistance, especially for use in traction batteries for electric cars and hybrid vehicles.
A more recent invention was a ceramic film developed as a separator which is temperature-resistant up to about 700 °C and is said to prevent explosive thermal runaway reliably.
Ceramic separators (membranes) are also used for solid-state-batteries.
Materials that can withstand the highly acidic and oxidative conditions are required here. Remember, the battery solvent is water here and the electrolyte is sulfuric acid. Extruded or sintered separators made of polyethylene, sintered polyvinyl chloride PVC or mats made of micro glass fiber fleece are possible here.

Ultracapacitor are different from batteries. There is no “battery chemistry” that requires chemically inert separators.
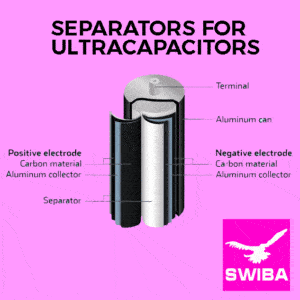
The separator in the Ultracapacitor need to be stable toward one electrolyte only. Often a cellulose filter is used a separator.
Cellulose is low-cost, highly permeable for the Ions and guaranteeing to catch up with the fast movement of the ions in super caps.
There are other synthetic PE/PP separators with modified, polar e.g. polyvinyl alcohol (PVA) surfaces that can be used in Ultracapacitor.
Here, in the strongly alkaline environment of the potassium hydroxide solution as the liquid aqueous electrolyte, separators made of polyamide and polyethylene or polypropylene combinations are predominantly used.
Nowadays, nonwovens are used almost exclusively here. Hydrophobic polymers can be made hydrophilic by fluorination or wetting agents, so that they eagerly absorb the electrolyte.
The requirements here are the same as for nickel-cadmium batteries, except that additional demands are placed on the battery separator. It must be able to reduce self-discharge.
This is achieved by functionalizing the nonwoven surface through chemical treatment. This can be surface treatment with acrylic acid or sulfonation.
Microporous films or nonwovens are used.
This type of battery mainly uses paper as a separator.
A non-academic perspective on the future of lithium-based batteries. Find more resources on Separators
Read about the industry lithium-ion battery prospective >>
In alkali-manganese batteries, nonwovens are predominantly used as separators. These usually consist of a mixture of polyvinyl alcohol microfibers (PVA) and cellulose. Laminates of nonwovens and membranes, such as cellophane, are also occasionally used.
The pore diameter must be small to avoid so-called growth through the separator by zinc dendrites, which leads to an internal short circuit. A low price of the material is also important.

Swiss Battery has developed with researchers from the ETH Ultrathin Membrane-Separators than can substitute expensive PTFE membranes. The thin membrane architecture allows der RFBs to operate in a high-power regime.
Read more about the
Ultrathin PBI-Membrane-Separators here >>

We live together for 8 hours per day, 5 days per week, so we spend a lot of time together. We do our best to make this a place where people can flourish, so they are happy and get along with other people easily.
We only hire people that add value to the team. We do not just hire great designers and marketers but people with passion to make awesome projects.
We want to surprise our clients with the end result. But not only that, we want to finish a project that will bless and grow the company of the project. That is why we ofter take a look at a project again and see how we can make it even better.
You can see right through us!